Abstract
The azurophil granule proteases neutrophil elastase (NE) and proteinase 3 (P3) have been established as effective immunotherapeutic targets in hematological malignancies. Expression of PR1, an HLA-A2 restricted nonameric peptide derived from NE and P3, has been demonstrated in acute myeloid leukemia (AML). We have successfully targeted PR1 in AML patients using PR1-peptide vaccine, anti-PR1/HLA-A2 antibody and PR1-specific cytotoxic T-lymphocytes (PR1-CTL). We have also identified the uptake and cross-presentation of NE and P3 by breast cancer, lung cancer and melanoma, which underlines their potential as immunotherapeutic targets in non-myeloid malignancies.
Cathepsin G is also an azurophil granule serine protease that is highly expressed by AML blasts and leukemic stem cells. Our previous studies showed a high level of efficacy in targeting AML blasts that express CG1 (FLLPTGAEA) using CG1-specific CTL (CG1-CTL), while sparing normal hematopoietic cells. However, two previous reports (Fujiwara et al., Clinical Cancer Research, 2005 and Gorodkiewicz et al., Analytical Biochemistry, 2012) and our published data (Alatrash G, Leukemia, 2017) have reported CG expression in lymphoid leukemia. We therefore hypothesized that CG is an effective immunotherapeutic target in lymphoid malignancies, specifically acute lymphoid leukemia (ALL).
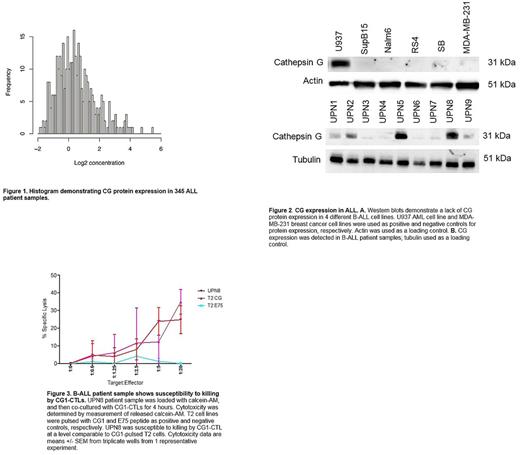
We first analyzed CG expression in ALL patient samples. Using reverse phase protein array (RPPA), we analyzed CG expression in ALL blasts from 345 adult ALL patient samples. We detected CG in 345 patient samples at levels comparable to G-CSF stimulated myeloid progenitors. The differential expression of CG in sorted ALL blasts ranged from -1.8030 to 5.4390, with a median value of 0.3506. In estimating CG levels, we found that more than 15% of our ALL samples expressed Log2 concentrations of CG between 0.3 and 0.4 (Figure 1). This was comparable to what we have previously reported in AML. We then wanted to determine whether the ALL-associated CG was due to uptake or endogenous expression. We performed PCR and western blotting and confirmed CG expression in 4 of 9 and 9 of 9 patient ALL samples, respectively (Figure 2). To assess whether CG is also taken up by ALL, we examined CG uptake by the B-ALL cell lines SB, RS4, Nalm6 and HMY (B-ALL). ALL cell lines were incubated with CG protein at increasing doses and durations. Intracellular staining of CG followed by flow cytometry analysis was used to determine uptake of CG by ALL cell lines. Our data show that all of the ALL cell lines take up CG; uptake correlated directly with increasing doses and time of incubation up to 24 hours.
Having characterized the expression of CG by ALL, we sought to determine its potential as an immunotherapeutic target, as we have previously shown for AML. In these experiments, CG1-specific CTLs were generated using standard dendritic cells methodology, interleukin-7 (IL-7), IL-2 and CG1-peptide pulsing. Expanded CG1-CTL were confirmed by staining cells with CG1 tetramer followed by flow cytometry analysis. We employed standard calcein-AM cytotoxicity assays to determine specific lysis. In these experiments, patient ALL sample UPN8 was pulsed with calcein-AM and then co-cultured with CG1-CTL for 4 hours. Our results indicated that UPN8 was effectively lysed by CG1-CTL, reaching a maximum specific lysis of ~30% at the highest effector:target ratio (Figure 3).
Overall, our analysis confirms CG endogenous expression and uptake by ALL. Furthermore, we conclude that CG is an effective target for immunotherapy in B-ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal